Learning Objectives
By the end of this section, you will be able to:
- Describe the fundamental properties of solutions
- Calculate solution concentrations using molarity
- Perform dilution calculations using the dilution equation
In preceding sections, we mostly focused on samples of matter that contain only one type of element or compound. However, mixtures—samples of matter containing two or more substances physically combined—are more commonly encountered in nature than are pure substances. In particular the homogeneous mixtures known as solutions are common in and outside of the laboratory. Much important chemistry occurs in solution, in the watery environment of our bodies, in the watery environment in nature, and in water and other solvents in industry and laboratories.

Solutions
We have previously defined solutions as homogeneous mixtures, meaning that the composition of the mixture (and therefore its properties) is uniform throughout its entire volume. Solutions occur frequently in nature and have also been implemented in many forms of manmade technology.
Often, a solution contains one component with a concentration that is significantly greater than that of all other components. This component is called the solvent and may be viewed as the medium in which the other components are dispersed, or dissolved. A solute is a component of a solution that is typically present at a much lower concentration than the solvent.
The relative amount of a given solute in a solution is known as its concentration. Solute concentrations are often described with qualitative terms such as dilute (of relatively low concentration) and concentrated (of relatively high concentration). But they can also be described quantitatively.
Concentration may be quantitatively assessed using a wide variety of measurement units. Percent (parts per hundred) come in several varieties, depending on the measurements employed: mass of solute/volume of solution, or volume of solute/volume of solution can be multiplied by 100 to yield these values.
Molarity (M) is a useful concentration unit for chemists. Molarity is defined as the number of moles of solute in exactly 1 liter (1 L) of the solution:
Example 1
A 355-mL soft drink sample contains 0.133 mol of sucrose (table sugar). What is the molar concentration of sucrose in the beverage?
Solution
Since the molar amount of solute and the volume of solution are both given, the molarity can be calculated using the definition of molarity. Per this definition, the solution volume must be converted from mL to L:
Test Yourself
A teaspoon of table sugar contains about 0.01 mol sucrose. What is the molarity of sucrose if a teaspoon of sugar has been dissolved in a cup of tea with a volume of 200 mL?
Answer
0.05 M
Example 2
How much sugar (mol) is contained in a sip (~10 mL) of the soft drink from Example 1?
Solution
In this case, we can rearrange the definition of molarity to isolate the quantity sought, moles of sugar. We then substitute the value for molarity that we derived in Example 1, 0.375 M:
[latex]M = \frac{\text{mol solute}}{\text{L solution}}[/latex]
[latex]\text{mol solute} = M \times \text{L solution}[/latex]
[latex]\text{mol solute} = 0.375 \;\frac{\text{mol sugar}}{\text{L}} \times (10 \;\text{mL} \times \frac{1 \text{L}}{1000 \;\text{mL}}) = 0.004 \;\text{mol sugar}[/latex]
Test Yourself
What volume (mL) of the sweetened tea described in Example 1 contains the same amount of sugar (mol) as 10 mL of the soft drink in this example?
Answer
80 mL
Example 3
Distilled white vinegar (Figure 2) is a solution of acetic acid, CH3CO2H, in water. A 0.500-L vinegar solution contains 25.2 g of acetic acid. What is the concentration of the acetic acid solution in units of molarity?

Solution
As in previous textbox shaded, the definition of molarity is the primary equation used to calculate the quantity sought. In this case, the mass of solute is provided instead of its molar amount, so we must use the solute’s molar mass to obtain the amount of solute in moles:
Test Yourself
Calculate the molarity of 6.52 g of CoCl2 (128.9 g/mol) dissolved in an aqueous solution with a total volume of 75.0 mL.
Answer
0.674 M
Example 4
How many grams of NaCl are contained in 0.250 L of a 5.30-M solution?
Solution
The volume and molarity of the solution are specified, so the amount (mol) of solute is easily computed as demonstrated in Example 2:
[latex]M = \;\frac{\text{mol solute}}{\text{L solution}}[/latex]
[latex]\text{mol solute} = M \times \text{L solution}[/latex]
[latex]\text{mol solute} = 5.30 \;\frac{\text{mol NaCl}}{\text{L}} \times 0.250 \;\text{L} = \underline{1.32}50 \;\text{mol NaCl with 3 sig figs}[/latex]
Finally, this molar amount is used to derive the mass of NaCl:
Test Yourself
How many grams of CaCl2 (110.98 g/mol) are contained in 250.0 mL of a 0.200-M solution of calcium chloride?
Answer
5.55 g CaCl2
Dilution of Solutions
Dilution is the process where the concentration of a solution is reduced by adding solvent.
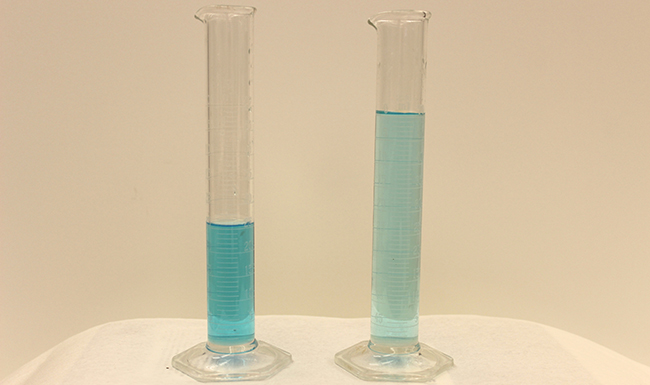
Dilution from a more concentrated stored “stock” solution is also a common means of preparing solutions of a desired concentration. For example, commercial pesticides are typically sold as solutions in which the active ingredients are far more concentrated than is appropriate for their application. Before they can be used on crops, the pesticides must be diluted. This is also a very common practice for the preparation of a number of common laboratory reagents (Figure 4).

A simple mathematical relationship can be used to relate the volumes and concentrations of a solution before and after the dilution process. According to the definition of molarity, the molar amount of solute in a solution is equal to the product of the solution’s molarity and its volume in liters:
Expressions like these may be written for a solution before and after it is diluted:
where the subscripts “1” and “2” refer to the solution before and after the dilution, respectively. Since the dilution process does not change the amount of solute in the solution,n1 = n2. Thus, these two equations may be set equal to one another:
This relation is commonly referred to as the dilution equation. Although we derived this equation using molarity as the unit of concentration and liters as the unit of volume, other units of concentration and volume may be used, so long as the units properly cancel per the factor-label method. Reflecting this versatility, the dilution equation is often written in the more general form:
where C and V are concentration and volume, respectively.

Use the simulation to explore the relations between solute amount, solution volume, and concentration and to confirm the dilution equation.
Example 6
What volume of 1.59 M KOH is required to prepare 5.00 L of 0.100 M KOH?
Solution
We are given the concentration of a stock solution, C1, and the volume and concentration of the resultant diluted solution, V2 and C2. We need to find the volume of the stock solution, V1. We thus rearrange the dilution equation in order to isolate V1:
[latex]V_2 = \frac{C_2V_2}{C_1}[/latex]
Since the concentration of the diluted solution 0.100 M is roughly one-sixteenth that of the stock solution (1.59 M), we would expect the volume of the stock solution to be about one-sixteenth that of the diluted solution, or around 0.3 liters. Substituting the given values and solving for the unknown volume yields:
[latex]V_1 = 0.314 \;\text{L}[/latex]
Thus, we would need 0.314 L of the 1.59-M solution to prepare the desired solution. This result is consistent with our rough estimate.
Test Yourself
What volume of a 0.575-M solution of glucose, C6H12O6, can be prepared from 50.00 mL of a 3.00-M glucose solution?
Answer
0.261 L
Key Concepts and Summary
Solutions are homogeneous mixtures. The concentration of a solution is a measure of the relative amount of solute in a given amount of solution. Concentrations may be measured using various units, with one very useful unit being Molarity. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution.
Key Equations
[latex]M = \frac{\text{mol solute}}{\text{L solution}}[/latex]
C1V1 = C2V2
Review-Reflect, Extend
Review-Reflect
1. Determine the molarity for each of the following solutions:
a) 0.444 mol of CoCl2 in 0.654 L of solution
b) 98.0 g of phosphoric acid, H3PO4, in 1.00 L of solution
c) 0.2074 g of calcium hydroxide, Ca(OH)2, in 40.00 mL of solution
2. Consider this question: What is the mass of the solute in 0.500 L of 0.30 M glucose, C6H12O6, used for intravenous injection?
a) Outline the steps necessary to answer the question.
b) Answer the question.
3. What volume of a 0.33M C12H22O11 solution can be diluted to prepare 25 mL of a solution with a concentration of 0.025 M?
4. What is the molarity of the diluted solution when each of the following solutions is diluted to the given final volume?
a) 1.00 L of a 0.250M solution of Fe(NO3)3 is diluted to a final volume of 2.00 L
b) 0.5000 L of a 0.1222M solution of C3H7OH is diluted to a final volume of 1.250 L
Extend
- It has been said that an alcoholic beverage contains the same amount of alcohol, whether it be a beer, a glass of wine, or a shot of distilled liquor. Choose 3 specific drinks (a particular brand and type of beverage) of this type and run the calculations, starting with the advertised percent alcohol and using that concentration and the volumes to arrive at your answer. Is the statement about their equivalence true, for the drinks you chose?
- In medical settings, dose is a commonly used unit. How is dose related to solution concentration? How is it different?
Answers
1. a) 0.679 M; b) 1.00 M; c) 0.06998 M;
2. a) determine the number of moles of glucose in 0.500 L of solution; determine the molar mass of glucose; determine the mass of glucose from the number of moles and its molar mass;
b) 27 g
3. 1.9 mL
4. a) 0.125 M; b) 0.04888 M;
Glossary
aqueous solution: solution for which water is the solvent
concentrated: qualitative term for a solution containing solute at a relatively high concentration
concentration: quantitative measure of the relative amounts of solute and solvent present in a solution
dilute: qualitative term for a solution containing solute at a relatively low concentration
dilution: process of adding solvent to a solution in order to lower the concentration of solutes
dissolved: describes the process by which solute components are dispersed in a solvent
molarity (M): unit of concentration, defined as the number of moles of solute dissolved in 1 liter of solution
solute: solution component present in a concentration less than that of the solvent
solvent: solution component present in a concentration that is higher relative to other components
