Learning Objectives
By the end of this module, you will be able to:
- Outline the historical development of chemistry
- Provide examples of the importance of chemistry in everyday life
- Distinguish between qualitative and quantitative observations
- Describe the differences between hypotheses and theories
- Classify examples illustrating macroscopic, microscopic, and symbolic domains
Throughout human history, people have tried to convert matter into more useful forms. These endeavors involved changing the shape of a substance without changing the substance itself. But as our knowledge increased, humans began to change the composition of the substances as well—clay was converted into pottery, hides were cured to make garments, copper ores were transformed into copper tools and weapons, and grain was made into bread.
Humans began to practice chemistry when they learned to control fire and use it to cook, make pottery, and smelt metals. Subsequently, they began to separate and use specific components of matter. A variety of drugs such as aloe, myrrh, and opium were isolated from plants. Dyes, such as indigo and Tyrian purple, were extracted from plant and animal matter. Alkalis were extracted from ashes, and soaps were prepared by combining these alkalis with fats.
Attempts to understand the behavior of matter extend back for more than 2500 years. As early as the sixth century BC, Greek philosophers discussed a system in which water was the basis of all things. You may have heard of the Greek postulate that matter consists of four elements: earth, air, fire, and water. Subsequently, an amalgamation of chemical technologies and philosophical speculations were spread from Egypt, China, and the eastern Mediterranean by alchemists, who endeavored to transform “base metals” such as lead into “noble metals” like gold, and to create elixirs to cure disease and extend life (Figure 1).

From alchemy came the historical progressions that led to modern chemistry: the isolation of drugs from natural sources, metallurgy, and the dye industry. Today, chemistry continues to deepen our understanding and improve our ability to harness and control the behavior of matter.
Chemistry: The Central Science
Chemistry is sometimes referred to as “the central science” due to its interconnectedness with a vast array of other STEM disciplines (STEM stands for areas of study in the science, technology, engineering, and math fields). Chemistry and the language of chemists play vital roles in biology, medicine, materials science, forensics, environmental science, and many other fields (Figure 2).
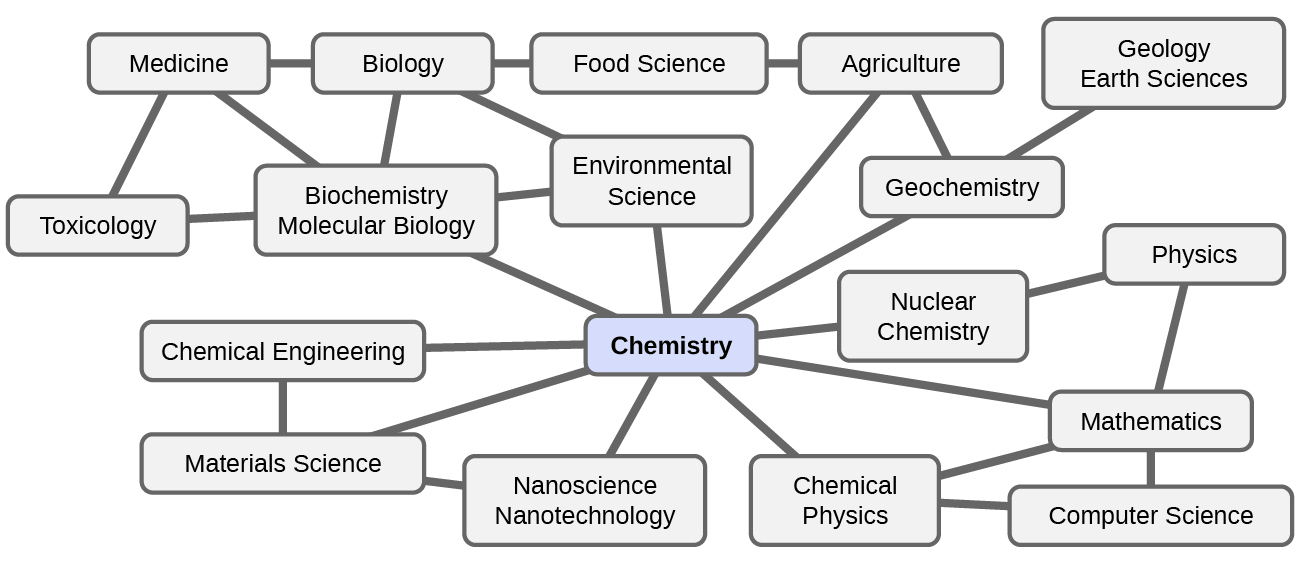
What are some changes in matter that are essential to daily life? Digesting and assimilating food, synthesizing polymers that are used to make clothing, containers, cookware, and credit cards, and refining crude oil into gasoline and other products are just a few examples. During this course you will discover many different examples of changes in the composition and structure of matter, how to classify these changes and how they occurred, their causes, the changes in energy that accompany them, and the principles and laws involved. As you learn about these things you will be learning chemistry, the study of the composition, properties, and interactions of matter.
The Scientific Method
It is important to understand that chemistry as a science is a procedure as well as sets of facts.
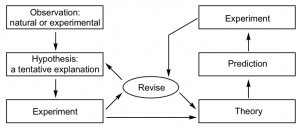
Chemistry is a science based on observation and experimentation. Doing chemistry involves attempting to answer questions and explain observations in terms of the laws and theories of chemistry, using procedures that are accepted by the scientific community. There is no single route to answering a question or explaining an observation, but there is an aspect common to every approach: Each uses knowledge based on experiments that can be reproduced to verify the results. Some routes involve a hypothesis, a tentative explanation of observations that acts as a guide for gathering and checking information. We test a hypothesis by experimentation, calculation, and/or comparison with the experiments of others and then refine it as needed.
If such a hypothesis turns out to be capable of explaining a large body of experimental data, it can reach the status of a theory. Scientific theories are well-substantiated, comprehensive, testable explanations of particular aspects of nature. Theories are accepted because they provide satisfactory explanations, but they can be modified if new data become available. The path of discovery that leads from question and observation to hypothesis to theory, combined with experimental verification of the hypothesis and any necessary modification of the theory, is called the scientific method (Figure 3).
Science can be either qualitative or quantitative. Qualitative observations are descriptive, they involve a description of the quality of an object. For example: sulfur is yellow, your math book is heavy, or that statue is pretty. Quantitative observations represent the specific amount of something, usually by counting or measuring it. Therefore, quantitative observations involve a quantity or number and units. As such, quantitative observations would include: 25 students in a class, 650 pages in a book, or a velocity of 66 miles per hour. Quantitative expressions are very important in science, and thus very important in chemistry.
Example 1
Identify each statement as either a qualitative observation or a quantitative observation.
a) Gold metal is yellow.
b) A ream of paper has 500 sheets in it.
c) The weather outside is snowy.
d) The temperature outside is 24 degrees Fahrenheit.
Solution
a) Because we are describing a physical property of gold, this statement is qualitative.
b) This statement mentions a specific amount, so it is quantitative.
c) The word snowy is a description of how the day is; therefore, it is a qualitative statement.
d) In this case, the weather is described with a specific quantity—the temperature. Therefore, it is quantitative.
The Domains of Chemistry
Chemists study and describe the behavior of matter and energy using three types of representations: macroscopic, microscopic, and symbolic. These three types provide different perspectives on chemical behavior. This framework for thinking about and describing chemistry is referred to as the Chemistry Triplet or as Johnstone’s Triangle, in reference to A.H. Johnstone who published these ideas in the Journal of Chemistry Education.[1]
Macro is a Greek word that means “large.” The macroscopic domain is familiar to us: It is the realm of everyday things that are large enough to be sensed directly by human sight or touch. In daily life, this includes the food you eat and the breeze you feel on your face. The macroscopic domain includes everyday and laboratory chemistry, where we observe and measure physical and chemical properties, or changes such as density, solubility, and flammability.
The microscopic domain of chemistry is almost always visited in the imagination. Micro comes from Greek and means “small.” Some aspects of the microscopic domains are visible through a microscope, such as a magnified image of graphite or bacteria. Viruses, for instance, are too small to be seen with the naked eye, but when we’re suffering from a cold, we’re reminded of how real they are.
However, most of the subjects in the microscopic domain of chemistry—such as atoms and molecules—are too small to be seen even with standard microscopes and often must be pictured in the mind. Other components of the microscopic domain include ions and electrons, protons and neutrons, and chemical bonds, each of which is far too small to see. This domain includes the individual metal atoms in a wire, the ions that compose a salt crystal, the changes in individual molecules that result in a color change, the conversion of nutrient molecules into tissue and energy, and the evolution of heat as bonds that hold atoms together are created.
The symbolic domain contains the specialized language used to represent components of the macroscopic and microscopic domains. Chemical symbols (such as those used in the periodic table), chemical formulas, and chemical equations are part of the symbolic domain, as are graphs and drawings. We can also consider calculations as part of the symbolic domain. These symbols play an important role in chemistry because they help interpret the behavior of the macroscopic domain in terms of the components of the microscopic domain.
One of the features that makes chemistry fascinating is the use of a domain that must be imagined (microscopic or symbolic) to explain behavior in a domain that can be observed (macroscopic).
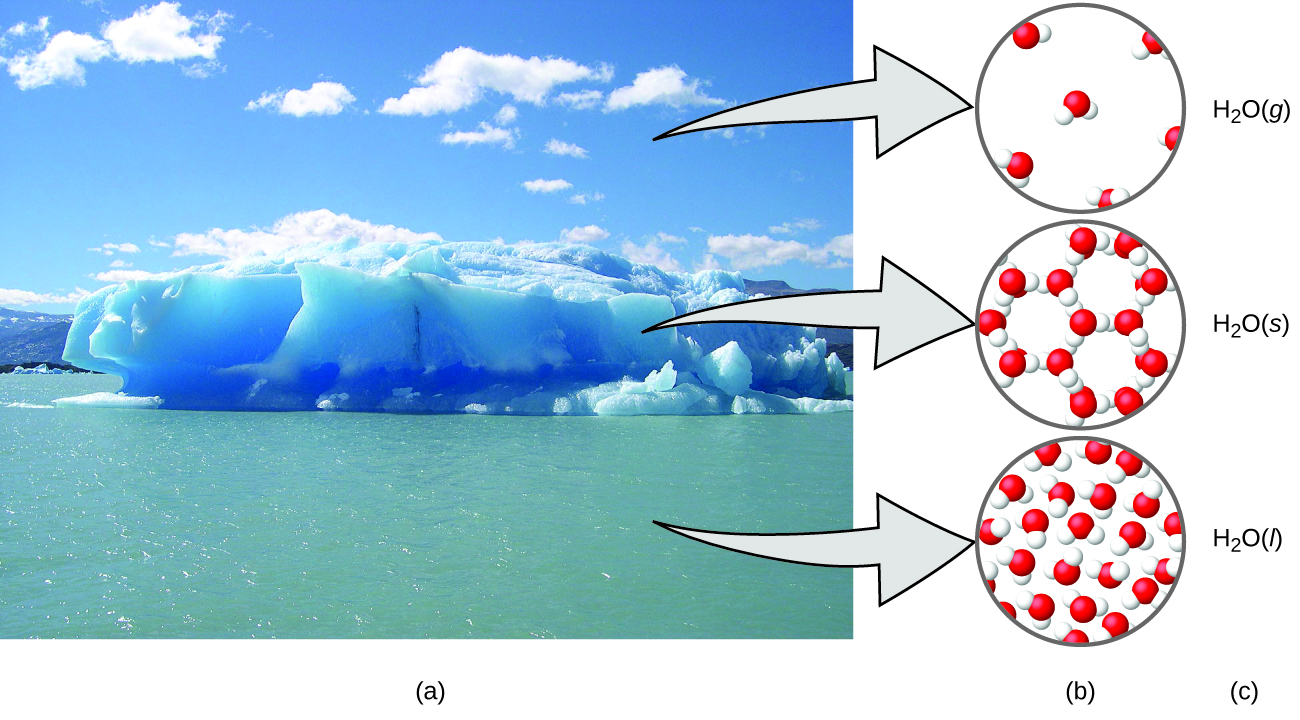
A helpful way to understand the three domains is via the essential and familiar substance of water. That water is a liquid at moderate temperatures, will freeze to form a solid at lower temperatures, and boil to form a gas at higher temperatures (Figure 4) are macroscopic observations. But some properties of water fall into the microscopic domain—what we cannot observe with the naked eye. The description of water as comprised of two hydrogen atoms and one oxygen atom, and the explanation of freezing and boiling in terms of attractions between these molecules, is within the microscopic domain. The formula H2O, which can describe water at either the macroscopic or microscopic levels, is an example of the symbolic domain. The abbreviations (g) for gas, (s) for solid, and (l) for liquid are also symbolic.
Key Concepts and Summary
Chemistry deals with the composition, structure, and properties of matter, and the ways by which various forms of matter may be interconverted. Thus, it occupies a central place in the study and practice of science and technology. Chemists use the scientific method to perform experiments, pose hypotheses and develop theories, so that we can better understand the natural world. To do so, chemists operate using the macroscopic, microscopic, and symbolic domains. Their work is critically important to our lives.
Review-Refresh/Extend
Review/Refresh
1. What essential features make for a scientific experiment?
2. What is the difference between a scientific hypothesis and a theory?
3. Look around you and identify 3 observations about your immediate environment that are qualitative descriptions. Then attempt to make 3 quantitative observations.
4. Identify each of the following statements as relating primarily to the macroscopic, microscopic or symbolic domains:
a) The pressure of a sample of gas is directly proportional to the temperature of the gas.
b) Matter consists of tiny particles that can combine in specific ratios to form substances with specific properties.
c) At a higher temperature, solids (such as salt or sugar) will dissolve better in water.
5. Identify each of the underlined items as a part of either the macroscopic domain, the microscopic domain, or the symbolic domain of chemistry. For those in the symbolic domain, indicate whether they are symbols for a macroscopic or a microscopic feature.
a) A certain molecule contains one H atom and one Cl atom.
b) Copper wire has a density of about 8 g/cm3.
c) The bottle contains 15 grams of Ni powder.
d) A sulfur molecule is composed of eight sulfur atoms.
Extend Your Understandings
- What is a scientific law? How does it differ from a scientific hypothesis or theory?
- Use Wikipedia to look up a familiar substance, such as copper, or silver, or nitrogen. Identify parts of the Wikipedia entry that describe the substance using each of the domains of Johnstone’s Triangle.
Answers to Review/Refresh
1. Simply stated, the scientific method includes three steps: (1) a hypothesis, (2) testing the hypothesis, and (3) refining the hypothesis.
2. A hypothesis is a tentative explanation that can be tested by an experiment. A theory is a comprehensive, established explanation that has already stood up well to experimentation.
3. Answers will vary, but qualitative observations are descriptive, e.g. “the wall is yellow” and “the room is comfortably warm.” Quantitative observations are typically measured with a measuring tool, e.g. “The room is 68 ºF'” or “The book I am reading has 336 pages.”
4. a) macroscopic b) microscopic c) macroscopic
5. a) symbolic, microscopic; b) macroscopic; c) symbolic, macroscopic; d) microscopic
Glossary
chemistry: study of is the study of matter and its properties, the changes that matter undergoes, and the energy associated with these changes.
hypothesis: tentative explanation of observations that acts as a guide for gathering and checking information
law: statement that summarizes a vast number of experimental observations, and describes or predicts some aspect of the natural world
macroscopic domain: realm of everyday things that are large enough to sense directly by human sight and touch
microscopic domain: realm of things that are much too small to be sensed directly
qualitative observations: they are descriptions of the quality of an object.
quantitative observations: they represent the specific amount of something; they involve a quantity or number and units.
scientific method: path of discovery that leads from question and observation to law or hypothesis to theory, combined with experimental verification of the hypothesis and any necessary modification of the theory
symbolic domain: specialized language used to represent components of the macroscopic and microscopic domains, such as chemical symbols, chemical formulas, chemical equations, graphs, drawings, and calculations
theory: well-substantiated, comprehensive, testable explanation of a particular aspect of nature
- Johnstone, A.H. J. Comp. Assist. Learn. 1991, 7, 701-703 ↵
